Learning Objectives
Learning Objectives
In this section, you will explore the following question:
- How do electrochemical gradients affect the active transport of ions and molecules across membranes?
Connection for AP® Courses
Connection for AP® Courses
If a substance must move into the cell against its concentration gradient, the cell must use free energy, often provided by ATP, and carrier proteins acting as pumps to move the substance. Substances that move across membranes by this mechanism, a process called active transport, include ions, such as Na+ and K+. The combined gradients that affect movement of an ion are its concentration gradient and its electrical gradient (the difference in charge across the membrane); together, these gradients are called the electrochemical gradient. To move substances against an electrochemical gradient requires free energy. The sodium-potassium pump, which maintains electrochemical gradients across the membranes of nerve cells in animals, is an example of primary active transport. The formation of H+ gradients by secondary active transport (cotransport) is important in cellular respiration and photosynthesis, and moving glucose into cells.
Information presented and the examples highlighted in the section support concepts and learning objectives outlined in Big Idea 2 of the AP® Biology Curriculum Framework. The learning objectives listed in the Curriculum Framework provide a transparent foundation for the AP® Biology course, an inquiry-based laboratory experience, instructional activities, and AP® exam questions. A learning objective merges required content with one or more of the seven science practices (SPs).
| Big Idea 2 | Biological systems use free energy and molecular building blocks to grow, to reproduce, and to maintain dynamic homeostasis. |
| Enduring Understanding 2.B | Growth, reproduction, and dynamic homeostasis require that cells create and maintain internal environments that are different from their external environments. |
| Essential Knowledge | 2.B.2 Growth and dynamic homeostasis are maintained by the constant movement of molecules across membranes. |
| Science Practice | 1.4 The student can use representations and models to analyze situations or solve problems qualitatively and quantitatively. |
| Learning Objective | 2.12 The student is able to use representations and models to analyze situations or solve problems qualitatively and quantitatively to investigate whether dynamic homeostasis is maintained by the active movement of molecules across membranes. |
The Science Practices Assessment Ancillary contains additional test questions for this section that will help you prepare for the AP exam. These questions address the following standards:
- [APLO 2.10]
- [APLO 2.17]
- [APLO 1.2]
- [APLO 3.24]
Active transport mechanisms require the use of the cell’s energy, usually in the form of adenosine triphosphate (ATP). If a substance must move into the cell against its concentration gradient—that is, if the concentration of the substance inside the cell is greater than its concentration in the extracellular fluid (and vice versa)—the cell must use energy to move the substance. Some active transport mechanisms move small-molecular-weight materials, such as ions, through the membrane. Other mechanisms transport much larger molecules.
Electrochemical Gradient
Electrochemical Gradient
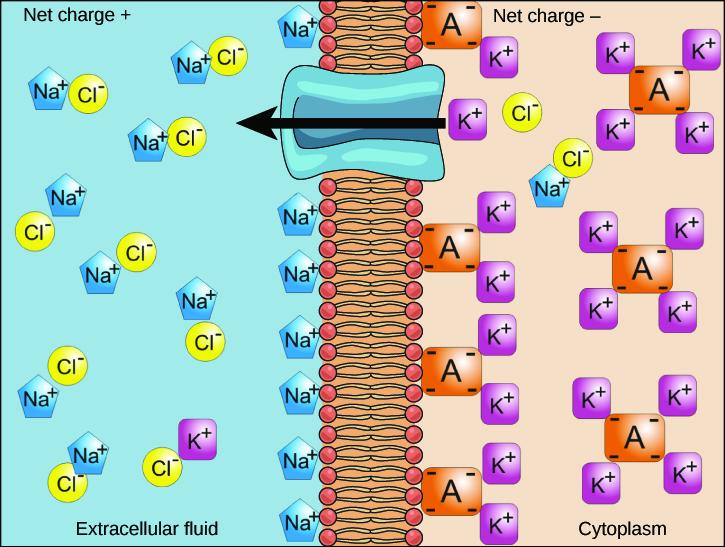
We have discussed simple concentration gradients—differential concentrations of a substance across a space or a membrane—but in living systems, gradients are more complex. Because ions move into and out of cells and because cells contain proteins that do not move across the membrane and are mostly negatively charged, there is also an electrical gradient, a difference of charge, across the plasma membrane. The interior of living cells is electrically negative with respect to the extracellular fluid in which they are bathed, and at the same time cells have higher concentrations of potassium (K+) and lower concentrations of sodium (Na+) than does the extracellular fluid. So in a living cell, the concentration gradient of Na+ tends to drive it into the cell, and the electrical gradient of Na+ (a positive ion) also tends to drive it inward to the negatively charged interior. The situation is more complex, however, for other elements, such as potassium. The electrical gradient of K+, a positive ion, also tends to drive it into the cell, but the concentration gradient of K+ tends to drive K+ out of the cell (Figure 5.17). The combined gradient of concentration and electrical charge that affects an ion is called its electrochemical gradient.
Visual Connection
If the pH outside the cell decreases, would you expect the amount of amino acids transported into the cell to increase or decrease?
- Transport of amino acids into the cell increases.
- Transport of amino acids into the cell stops.
- Transport of amino acids into the cell is not affected by pH.
- Transport of amino acid into the cell decreases.
Moving Against a Gradient
Moving Against a Gradient
To move substances against a concentration or electrochemical gradient, the cell must use energy. This energy is harvested from ATP generated through the cell’s metabolism. Active transport mechanisms, collectively called pumps, work against electrochemical gradients. Small substances constantly pass through plasma membranes. Active transport maintains concentrations of ions and other substances needed by living cells in the face of these passive movements. Much of a cell’s supply of metabolic energy may be spent maintaining these processes. Most of a red blood cell’s metabolic energy is used to maintain the imbalance between exterior and interior sodium and potassium levels required by the cell. Because active transport mechanisms depend on a cell’s metabolism for energy, they are sensitive to many metabolic poisons that interfere with the supply of ATP.
Two mechanisms exist for the transport of small-molecular-weight material and small molecules. Primary active transport moves ions across a membrane and creates a difference in charge across that membrane, which is directly dependent on ATP. Secondary active transport describes the movement of material that is due to the electrochemical gradient established by primary active transport, which does not directly require ATP.
Carrier Proteins for Active Transport
Carrier Proteins for Active Transport
An important membrane adaption for active transport is the presence of specific carrier proteins or pumps to facilitate movement; there are three types of these proteins or transporters (Figure 5.18). A uniporter carries one specific ion or molecule. A symporter carries two different ions or molecules, both in the same direction. An antiporter also carries two different ions or molecules, but in different directions. All of these transporters can also transport small, uncharged organic molecules, such as glucose. These three types of carrier proteins are also found in facilitated diffusion, but they do not require ATP to work in that process. Some examples of pumps for active transport are Na+-K+ ATPase, which carries sodium and potassium ions, and H+-K+ ATPase, which carries hydrogen and potassium ions. Both of these are antiporter carrier proteins. Two other carrier proteins are Ca2+ ATPase and H+ ATPase, which carry only calcium and only hydrogen ions, respectively. Both are pumps.
Everyday Connection for AP® Courses
The primary active transport that functions with the active transport of sodium and potassium allows secondary active transport to occur. The second transport method is still considered active because it depends on the use of energy as does primary transport (illustrative example).
One of the most important pumps in animal cells is the sodium-potassium pump (Na+-K+ ATPase), which maintains the electrochemical gradient (and the correct concentrations of Na+ and K+) in living cells. The sodium-potassium pump moves K+ into the cell while moving Na+ out at the same time, at a ratio of three Na+ for every two K+ ions moved in. The Na+-K+ ATPase exists in two forms, depending on its orientation to the interior or exterior of the cell and its affinity for either sodium or potassium ions. The process consists of the following six steps:
- With the enzyme oriented toward the interior of the cell, the carrier has a high affinity for sodium ions. Three ions bind to the protein.
- The protein carrier hydrolyzes ATP, and a low-energy phosphate group attaches to it.
- As a result, the carrier changes shape and reorients itself toward the exterior of the membrane. The protein’s affinity for sodium decreases, and the three sodium ions leave the carrier.
- The shape change increases the carrier’s affinity for potassium ions, and two such ions attach to the protein. Subsequently, the low-energy phosphate group detaches from the carrier.
- With the phosphate group removed and potassium ions attached, the carrier protein repositions itself toward the interior of the cell.
- The carrier protein, in its new configuration, has a decreased affinity for potassium, and the two ions are released into the cytoplasm. The protein now has a higher affinity for sodium ions, and the process starts again.
Several things have happened as a result of this process. At this point, there are more sodium ions outside the cell than inside, and more potassium ions inside than out. For every three ions of sodium that move out, two ions of potassium move in. This results in the interior being slightly more negative relative to the exterior. This difference in charge is important in creating the conditions necessary for the secondary process. Therefore, the sodium-potassium pump is an electrogenic pump (a pump that creates a charge imbalance), contributing to the membrane potential.
What will happen to the opening of the sodium-potassium pump if no ATP is present in a cell?
- It will remain facing the extracellular space, with sodium ions bound.
- It will remain facing the extracellular space, with potassium ions bound.
- It will remain facing the cytoplasm, but no sodium ions would bind.
- It will remain facing the cytoplasm, with sodium ions bound.
Link to Learning
Visit the site to see a simulation of active transport in a sodium-potassium ATPase.
- ATP is required to move sodium ions against their concentration gradient outside the cell.
- ATP is required to allow entry of potassium ions inside the cell.
- ATP is required to allow entry of sodium ions inside the cell.
- ATP is required to release potassium ions outside the cell.
Science Practice Connection for AP® Courses
Activity
Create a representation/diagram, or use the model you constructed of the plasma cell membrane, to explain how the sodium-potassium pump contributes to the net negative change of the interior of an animal nerve cell.
Think About It
If the pH outside the cell decreases, would you expect the amount of amino acids and glucose transported into the cell to increase or decrease? Justify your reasoning.
Secondary Active Transport (Cotransport)
Secondary Active Transport (Cotransport)
Secondary active transport brings sodium ions, and possibly other compounds, into the cell. As sodium ion concentrations build outside the plasma membrane because of the action of the primary active transport process, an electrochemical gradient is created. If a channel protein exists and is open, the sodium ions will be pulled through the membrane. This movement is used to transport other substances that can attach themselves to the transport protein through the membrane (Figure 5.20). Many amino acids, as well as glucose, enter a cell this way. This secondary process is also used to store high-energy hydrogen ions in the mitochondria of plant and animal cells for the production of ATP. The potential energy that accumulates in the stored hydrogen ions is translated into kinetic energy as the ions surge through the channel protein ATP synthase, then that energy is used to convert ADP into ATP.
Visual Connection
Injection of a potassium solution into a person’s blood is lethal. Potassium is used in capital punishment and euthanasia. Why do you think a potassium solution injection is lethal?
- Excess potassium disrupts the membrane components.
- Excess potassium increases action potential generation, leading to uncoordinated organ activity.
- Potassium dissipates the electrochemical gradient in cardiac muscle cells, preventing them from contracting.
- Potassium creates a new concentration gradient across the cell membrane, preventing sodium from leaving the cell.
Short Answer
Short Answer
Explain how the sodium-potassium pump contributes to the net negative charge of the interior of the cell and the formation of an electrochemical gradient across the plasma cell membrane.
Explain why it is important that there are different types of proteins in plasma membranes for the transport of materials into and out of the cell.
Justify the claim that ions have a difficult time getting through plasma membranes despite their small size.
Explain why the active movement of molecules across membranes must function continuously.
Explain how the sodium-potassium pump makes the interior of the cell negatively charged.
Describe how the electrical gradient and concentration gradient is established across a plasma cell membrane.
Disclaimer
This section may include links to websites that contain links to articles on unrelated topics. See the preface for more information.