Learning Objectives
Learning Objectives
In this section, you will explore the following questions:
- What is entropy?
- What is the difference between the first and second laws of thermodynamics?
Connection for AP® Courses
Connection for AP® Courses
In studying energy, scientists use the term system to refer to the matter and its environment involved in energy transfers, such as an ecosystem. Even single cells are biological systems and all systems require energy to maintain order. The more ordered a system, the lower its entropy. Entropy is a measure of the disorder of the system. (Think of your bedroom as a system. On Sunday evening, you throw dirty clothes in the laundry basket, put books back on the shelves, and return dirty dishes to the kitchen. Cleaning your room requires an input of energy. What gradually happens as the week progresses? You guessed it: entropy.) All biological systems obey the laws of chemistry and physics, including the laws of thermodynamics that describe the properties and processes of energy transfer in systems. The first law states that the total amount of energy in the universe is constant; energy cannot be created or destroyed, but it can be transformed and transferred. The second law states that every energy transfer involves some loss of energy in an unusable form, such as heat energy, resulting in a more disordered system, e.g., your bedroom over the course of a week. Thus, no energy transfer is completely efficient. We will explore how free energy is stored, transferred, and used in more detail when we study photosynthesis and cellular respiration.
Information presented and the examples highlighted in the section, support concepts and Learning Objectives outlined in Big Idea 2 of the AP® Biology Curriculum Framework. The Learning Objectives listed in the Curriculum Framework provide a transparent foundation for the AP® Biology course, an inquiry-based laboratory experience, instructional activities, and AP® Exam questions. A Learning Objective merges required content with one or more of the seven Science Practices.
| Big Idea 2 | Biological systems utilize free energy and molecular building blocks to grow, to reproduce, and to maintain dynamic homeostasis. |
| Enduring Understanding 2.A | Growth, reproduction, and maintenance of living systems require free energy and matter. |
| Essential Knowledge | 2.A.1 All living systems require constant input of free energy. |
| Science Practice | 6.2 The student can construct explanations of phenomena based on evidence produced through scientific practices. |
| Learning Objective | 2.1 The student is able to explain how biological systems use free energy based on empirical data that all organisms require constant energy input to maintain organization, to grow, and to reproduce. |
The Science Practices Assessment Ancillary contains additional test questions for this section that will help you prepare for the AP® exam. These questions address the following standards:
- [APLO 2.1]
- [APLO 2.2]
- [APLO 2.4]
- [APLO 4.16]
- [APLO 2.3]
Thermodynamics refers to the study of energy and energy transfer involving physical matter. The matter and its environment relevant to a particular case of energy transfer are classified as a system, and everything outside of that system is called the surroundings. For instance, when heating a pot of water on the stove, the system includes the stove, the pot, and the water. Energy is transferred within the system—between the stove, pot, and water. There are two types of systems: open and closed. An open system is one in which energy can be transferred between the system and its surroundings. The stovetop system is open because heat can be lost into the air. A closed system is one that cannot transfer energy to its surroundings.
Biological organisms are open systems. Energy is exchanged between them and their surroundings, as they consume energy-storing molecules and release energy to the environment by doing work. Like all things in the physical world, energy is subject to the laws of physics. The laws of thermodynamics govern the transfer of energy in and among all systems in the universe.
The First Law of Thermodynamics
The First Law of Thermodynamics
The first law of thermodynamics deals with the total amount of energy in the universe. It states that this total amount of energy is constant. In other words, there has always been, and always will be, exactly the same amount of energy in the universe. Energy exists in many different forms. According to the first law of thermodynamics, energy may be transferred from place to place or transformed into different forms, but it cannot be created or destroyed. The transfers and transformations of energy take place around us all the time. Light bulbs transform electrical energy into light energy. Gas stoves transform chemical energy from natural gas into heat energy. Plants perform one of the most biologically useful energy transformations on earth—that of converting the energy of sunlight into the chemical energy stored within organic molecules, as shown in Figure 6.2. Some examples of energy transformations are shown in Figure 6.11.
The challenge for all living organisms is to obtain energy from their surroundings in forms that they can transfer or transform into usable energy to do work. Living cells have evolved to meet this challenge very well. Chemical energy stored within organic molecules, such as sugars and fats, is transformed through a series of cellular chemical reactions into energy within molecules of ATP. Energy in ATP molecules is easily accessible to do work. Examples of the types of work that cells need to do include building complex molecules, transporting materials, powering the beating motion of cilia or flagella, contracting muscle fibers to create movement, and reproduction.
The Second Law of Thermodynamics
The Second Law of Thermodynamics
A living cell’s primary tasks of obtaining, transforming, and using energy to do work may seem simple. However, the second law of thermodynamics explains why these tasks are harder than they appear. None of the energy transfers we’ve discussed, along with all energy transfers and transformations in the universe, is completely efficient. In every energy transfer, some amount of energy is lost in a form that is unusable. In most cases, this form is heat energy. Thermodynamically, heat energy is defined as the energy transferred from one system to another that is not doing work. For example, when an airplane flies through the air, some of the energy of the flying plane is lost as heat energy due to friction with the surrounding air. This friction actually heats the air by temporarily increasing the speed of air molecules. Likewise, some energy is lost as heat energy during cellular metabolic reactions. This is good for warm-blooded creatures like us, because heat energy helps to maintain our body temperature. Strictly speaking, no energy transfer is completely efficient, because some energy is lost in an unusable form.
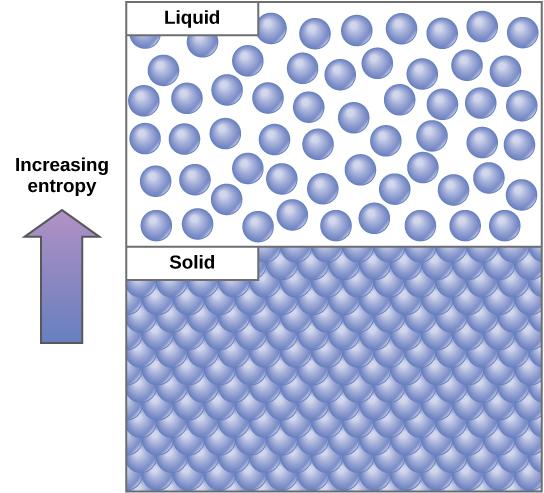
An important concept in physical systems is that of order and disorder, also known as randomness. The more energy that is lost by a system to its surroundings, the less ordered and more random the system. Scientists refer to the measure of randomness or disorder within a system as entropy. High entropy means high disorder and low energy (Figure 6.12). To better understand entropy, think of a student’s bedroom. If no energy or work were put into it, the room would quickly become messy. It would exist in a very disordered state, one of high entropy. Energy must be put into the system, in the form of the student doing work and putting everything away, in order to bring the room back to a state of cleanliness and order. This state is one of low entropy. Similarly, a car or house must be constantly maintained with work in order to keep it in an ordered state. Left alone, the entropy of the house or car gradually increases through rust and degradation. Molecules and chemical reactions have varying amounts of entropy as well. For example, as chemical reactions reach a state of equilibrium, entropy increases, and as molecules at a high concentration in one place diffuse and spread out, entropy also increases.
Scientific Method Connection
Transfer of Energy and the Resulting Entropy
Set up a simple experiment to understand how energy is transferred and how a change in entropy results.
- Take a block of ice. This is water in solid form, so it has a high structural order. This means that the molecules cannot move very much and are in a fixed position. The temperature of the ice is 0°C. As a result, the entropy of the system is low.
- Allow the ice to melt at room temperature. What is the state of molecules in the liquid water now? How did the energy transfer take place? Is the entropy of the system higher or lower? Why?
- Heat the water to its boiling point. What happens to the entropy of the system when the water is heated?
All physical systems can be thought of in this way: Living things are highly ordered, requiring constant energy input to be maintained in a state of low entropy. As living systems take in energy-storing molecules and transform them through chemical reactions, they lose some amount of usable energy in the process, because no reaction is completely efficient. They also produce waste and by-products that aren’t useful energy sources. This process increases the entropy of the system’s surroundings. Since all energy transfers result in the loss of some usable energy, the second law of thermodynamics states that every energy transfer or transformation increases the entropy of the universe. Even though living things are highly ordered and maintain a state of low entropy, the entropy of the universe in total is constantly increasing due to the loss of usable energy with each energy transfer that occurs. Essentially, living things are in a continuous uphill battle against this constant increase in universal entropy.
Science Practice Connection for AP® Courses
Think About It
- Imagine a large ant colony with an elaborate nest, containing many tunnels and passageways. Now imagine that an earthquake shakes the ground and demolishes the nest. Did the ant nest have higher entropy before or after the earthquake? What can the ants do to restore their nest to close to its original amount of entropy? Explain your answers.
- Energy transfers take place constantly in everyday activities. Think of two scenarios: cooking on a stove and driving a car. Explain how the second law of thermodynamics applies to these two scenarios.