Engage: Arrangement of Grocery Store Items
If you went into a grocery store, how would you find a loaf of bread or a gallon of milk?
Where would you find the ice cream?
What about your favorite cereal, an apple, or an orange?
How are items arranged in a grocery store?
How successful were you at finding the correct aisle?
Grocery stores are organized in a manner that helps customers easily find items.
Similar items are grouped together. Canned vegetables are placed in the canned goods section of the store, while fresh vegetables are found in the produce department.
Think about how the items are arranged on the shelf.
Many times smaller bags of items like sugar, flour, and pet food are located on the top or middle shelves, while larger, heavier bags are found on the bottom shelves.
Explore: What is the Periodic Table and How Was It Developed?
It is easy to move up and down the aisles and notice how similar items are grouped together in a grocery store.
Organizing information is beneficial. Tables, charts, and graphs are used to arrange information for easy reference.
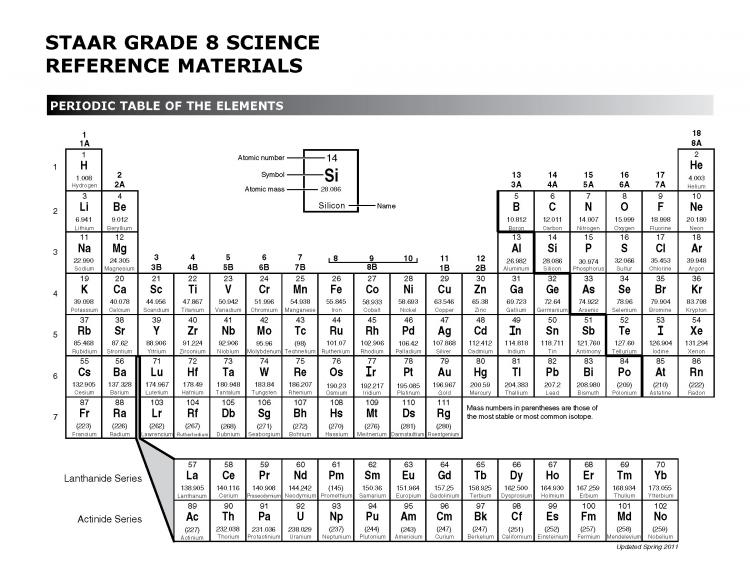
Scientists have organized the elements into a table based on their properties. This table is called the Periodic Table. Dimitri Mendeleev, a Russian scientist, is credited with the creation of the Periodic Table. Mendeleev based his arrangement of elements on both increasing atomic mass and similar properties. Today, we continue to use the Periodic Table as a reference to help identify the physical and chemical properties of elements.
Click on the Periodic Table to watch a video about the creation of the Periodic Table.
Listen for the answers to the following questions:
- How did Mendeleev arrange the elements on his table?
- How does the Periodic Table get its unique shape?
*Note* Video will open in a new window. Close the window to return to this resource.
Explain 1: What's in the Box?
Does the Periodic Table look like a bunch of letters and numbers to you?
The Periodic Table can provide a wealth of information if it is interpreted properly.
Click on the video below to understand and interpret the arrangement of elements in the Periodic Table. Be sure to click on the "full screen" icon.
Record the information in your notebook or on the Periodic Table handout. Find a copy of the Periodic Table and the Periodic Table handout in the “View Related Items” section below.
In this video, learn how to interpret the information found in each element's designated space.
Explain 2: Across the Periods
In the following videos, learn how to
- recognize periods and
- determine the number of energy levels for an element.
Record the information in your notebook or on the Periodic Table handout. Find a copy of the Periodic Table and the Periodic Table handout in the “View Related Items” section below.
Explain 3: What's the Pattern? Metals, Nonmetals, and Metalloids
In the following video, learn how to determine if an element is a metal, nonmetal, or metalloid based on its placement in the Periodic Table.
Record the information about the properties of metals, nonmetals, and metalloids in your notebook or on the Periodic Table handout. Find a copy of the Periodic Table and the Periodic Table handout in the “View Related Items” section below.
Explain 4: Groups Go Up!
Elements in the Periodic Table are commonly classified into groups or families. In the following videos, learn how to
- recognize groups on the Periodic Table,
- use the Periodic Table to determine valence electrons, and
- identify elements with similar properties.
Elaborate: Periodic Table Interpretations
Complete the following tasks using the information that you have learned.
Use your Periodic Table and the information you have learned about interpreting the Periodic Table to complete the activities.
To retake the quiz, reload the page and then select "No" when the "Resume Quiz" dialog box appears.
Evaluate: Periodic Table Check
Use your knowledge and your Periodic Table to interpret the arrangement of the Periodic Table. To retake the quiz, reload the page and then select "No" when the "Resume Quiz" dialog box appears.
Teacher Notes
In this lesson, students will interpret the arrangement of the Periodic Table, including groups and periods, to explain how properties are used to classify elements (TEKS 8.5A).
| Lesson Cycle | Activity |
| Engage | Students read about the organization of grocery store items and arrange several items in a grocery store. Later in the lesson, students will compare the arrangement of a grocery store to the Periodic Table. |
| Explore | As an introduction, students watch a video that discusses how Mendeleev developed the Periodic Table based on chemical properties. Students should focus on how Mendeleev arranged the elements on his table and the unique shape of Periodic Table. If students are unfamiliar with physical and chemical properties, encourage students to revisit Project Share resources for TEKS 8(5)(E), Physical or Chemical Change Interactive. It is assumed that students are familiar with physical and chemical properties and terminology such as malleable, ductile, and luster. |
|
Explain 1-4 |
Students learn how to interpret the Periodic Table. Students complete notes to describe characteristics of the groups and periods during or immediately after watching the videos. It is suggested that the teacher stop or direct students to pause the videos in order to record notes. Additional videos are provided to help students’ understanding of the Periodic Table. It is assumed that students have had exposure to the atomic structure, including protons, electrons, and energy levels, and understand that in an uncharged atom the number of protons and electrons are the same. Preteaching will be necessary if students are unfamiliar with the atomic structure. Some knowledge of elements reacting to form compounds would be beneficial, but not necessary. Students should be familiar with terms, including reactivity and valence electrons. In Explain 1, students learn to identify important information about each element. In Explain 2, students recognize that periods are horizontal rows and that elements in the same period have the same number of energy levels. Metals, nonmetals, and metalloids; their properties; and Periodic Table placement are discussed in Explain 3. Explain 4 includes defining groups, determining valence electrons, and discussing how elements in the same group have similar properties. |
| Elaborate | Students work through interactive activities using their knowledge of the Periodic Table. Students then create a nonlinguistic representation to remember the difference between a group and a period. Students also compare a grocery store to the Periodic Table. |
| Evaluate | Students review their acquired knowledge about the Periodic Table by answering multiple choice questions. |
Video Quick Reference Guide
| Video Name | Video Location | Concept |
|
How to One What's in the Box? |
Explain 1 | Each element has its own designated space on the Periodic Table that contains important information. |
|
How to Two Periods |
Explain 2 | Periods are horizontal rows located on the Periodic Table. |
|
How to Three Energy Levels |
Explain 2 | Elements in the same periods have the same number of energy levels. |
|
How to Four Metals, Nonmetals, and Metalloids |
Explain 3 | An element can be identified as a metal, nonmetal, or metalloid based on its placement on the Periodic Table. |
|
How to Five Groups |
Explain 4 | The vertical columns in the Periodic Table are called groups. |
|
How to Six Valence Electrons |
Explain 4 | The Periodic table can help easily determine the valence electrons for many elements. |
|
How to Seven Properties of Groups |
Explain 4 | Elements in the same groups have similar properties. |