Engage: On the Move!
Have you ever wondered why there are warning labels on spray cans about leaving them in the heat? What might happen? How can a gas fill up a balloon and keep it inflated for a few days? The answers to these questions can be found in the behavior of gas particles.
Take a moment to view this gas particles simulation. (Remember that models, as representations of an object or process, are limited.)
Make a few observations in your science notebook about the movement of the gas particles you see. Adjust the various controls on the screen to see how the behavior of the gas particles changes. Include a simple drawing of what you observe in your science notebook entry. When you finish, be ready to further explore the motion of gas particles in order to find out the reason for those warning labels on spray cans!
Explore: The Mystery of Gases
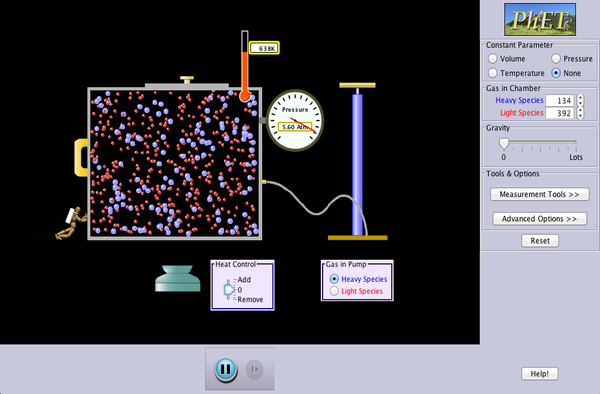
We are going to continue our study of gas particle behavior by exploring a second simulation. As you change the variables, consider the following questions and record your thoughts and observations for each one in your science notebook:
- Describe the motion of the gas particles.
- What affects the direction of each particle?
- Is there anything else present in the box with the gas particles?
- What happens when gas particles crash into each other?
Click the image below to proceed.
(You may be prompted to install Java before running the simulation.)
Explain: Kinetic Molecular Theory
The "rules" that explain how gas particles move are part of what's called the Kinetic Molecular Theory or KMT. The word kinetic refers to motion, so kinetic energy is the energy of the motion of an object. In this slideshow, you will see each postulate (rule) of the KMT and how it relates to the motion of gas particles you have observed already. Be ready to take notes in your science notebook as you work through the video.
Elaborate: Predicting Not so Random Behaviors
Based on what you now know about the KMT, how do you think increasing the temperature of a gas will affect the motion of the particles? Why does changing temperature affect the motion the way it does? Record your thoughts as you consider this question. If you need to refer back to the simulation from the Explore section, go ahead and do so.
Use your knowledge of the Kinetic Molecular Theory to explain the behavior of gas particles in each of the scenarios cited below.
- Why does a gas such as helium compress into a tank?
- Why can a small tank of helium gas fill so many balloons?
- Why would a pressurized spray can possibly explode if left in a hot car?
- Why can you smell a burning candle throughout an entire house?
- Why does an inflated pool toy seem flat after sitting in an air conditioned space?
Record your answers and ideas in your science notebook.
Evaluate: Check Your Understanding
Teacher Notes
This resource is a compilation of text, videos, and other elements to create a scaffolded 5E learning experience for students. This is meant for Tier I instruction under the Response to Intervention (RtI) model for high school chemistry, TEKS (9)(C).
Be sure to review the entire resource and the related items before assigning it to, or working through it with, your students to check for prerequisite knowledge and skills as well as differentiation needs.
This resource can be used for instruction in a variety of ways.
- Use with a single computer and projector; this can be delivered in a traditional classroom.
- Use with a combination of teacher computer/projector and individual student computers (in either a computer lab or other 1:1 environment).
- Assign to students as work to do outside of the school day as part of a "flipped classroom" to allow for application, practice, and additional support during the school day.
- Use with students as tutorials.
- Share with parents to inform them about what their child is learning in school.
- Use with students who are unable to participate in the traditional classroom environment.
Engage
Students have the opportunity to interact with a simulation that shows the movement of gas particles. It is important for them to discover on their own details about how the particles are moving. While students have learned about gas particles for several years, the details of their motion may not be at the forefront of what they know or remember. Encourage students to manipulate the simulation and to record detailed observations.
Explore
Students will now be able to explore a more detailed simulation within the context of the whipped cream spray can introduced through the cartoon. Resist the urge to answer students' questions or to explain what is happening. It is very important that students explore this concept as they seek an explanation for the cartoon character's concern over the spray can. If students are working in a classroom setting, it would be good for them to share their recorded observations and predictions with a partner after viewing this portion of the resource.
Explain
The tutorial slides take students step by step through the Kinetic Molecular Theory using text, graphics and references to the simulations they have seen prior to this part of the resource. Students should be encouraged to record the postulates in their notes. A few basic vocabulary terms have been provided at the beginning of the tutorial to support ELLs.
A few misconceptions that have been addressed in "Explain" are that students often think there is air between gas particles. They struggle to realize that it is just empty space. A comparison to the empty space in the universe or the empty space in an atom would help them make this connection. Students also can have the misconception that gases are not uniformly spread out in a container and that somehow the motion or direction of the particles is guided or controlled. Rather, it is important to clarify that the motion is influenced by the temperature, but only to the extent that an increase in the temperature of a gas signifies an increase in the gas particles' speed, not that the location of particles or the direction of motion is determined by the temperature.
Elaborate
Elaborate challenges students to extend their thinking to daily situations or examples. It is vital that students make connections between what they have learned and what they see on a daily basis. Encourage students to record their answers to these questions and then to share with a partner, their table, or the class. This dialogue will be crucial in helping solidify their knowledge, extend their learning, and clarify any last misconceptions.
Evaluate
This five-question quiz allows students to check their understanding and identify any parts of the Kinetic Molecular Theory that they may still have questions about. Students should be encouraged to record any doubts or questions so that those can be addressed in class.