Science Practice Challenge Questions
3.1 Synthesis of Biological Macromolecules
The capture of radiant energy through the conversion of carbon dioxide and water into carbohydrates is the engine that drives life on Earth. Ribose, C5H10O5, and hexose, C6H12O6, form stable five- and six-carbon rings.
The numbering of the carbons on these rings is important in organizing our description of the role these molecules play in biological energy transfer and information storage and retrieval. Glycolysis is a sequence of chemical reactions that convert glucose to two three-carbon compounds called pyruvic acid.
A. Create visual representations to show how when bonds in the glucose molecules are broken between carbon number 1 and the oxygen atom and between carbons 3 and 4, two molecules of pyruvic acid are produced.
Several enzymes in the cell are involved in converting glucose to pyruvic acid. These enzymes are proteins whose amino acid sequences provide these functions. This protein structure is information that was inherited from the cell’s parent, and is stored in deoxyribonucleic acid (DNA). The “deoxyribo” component of that name is a shorthand for 2-deoxyribose.
B. Create a visual representation of 2-deoxyribose, 5-phosphate by replacing the OH at carbon 2 with a hydrogen atom and replacing the OH at carbon 5 with a hydrogen phosphate ion, HPO32-, whose structure is shown in problem AP3.2. Use your representation to show that both phosphorylation (the addition of a phosphate ion) at carbon 5 and removal of the hydroxide at carbon 2 produce water molecules in an aqueous solution where hydrogen ions are abundant.
DNA is a polymer formed from a chain with repeated 2-deoxyribose, 5-phosphate molecules.
C. Create a visual representation of three 2-deoxyribose, 5-phosphate molecules forming a chain in which an oxygen atom in the phosphate that is attached to the 5-carbon replaces the OH on the 3-carbon of the next ribose sugar.
3.2 Carbohydrates
Cells are bounded by membranes composed of phospholipids. A phospholipid consists of a pair of fatty acids that may or may not have carbon-carbon double bonds, fused at the carboxylic acid with a three-carbon glycerol that is terminated by a phosphate, as shown in the figure below. Most cell membranes comprise two phospholipid layers with the hydrophilic phosphate ends of each molecule in the outer and inner surfaces. The hydrophobic chains of carbon atoms extend into the space between these two surfaces.
The exchange of matter between the interior of the cell and the environment is mediated by this membrane with selective permeability.
A. Pose questions that identify
- the important characteristics of this lipid bilayer structure
- the molecules that must be acquired from the environment and eliminated from the cell
- relationships between the structures of these molecules and the structure of the bilayer
Because the plasma cell membrane has both hydrophilic and hydrophobic properties, few types of molecules possess structures that allow them to pass between the interior of the cell and the environment through passive diffusion. The fluidity of the membrane affects passive transport, and the incorporation of other molecules in the membrane, in particular cholesterols, has a strong effect on its fluidity. Fluidity is also affected by temperature.
Measurements of the speed of movement of oxygen molecules, O2, through three types of membranes were made (Widomska et al., Biochimica et Biophysica Acta, 1,768, 2007) and compared with the speed of movement of O2 through water. These measurements were carried out at four different temperatures. One type of membrane was obtained from the cells in the eyeball of a calf (lens lipid). Synthetic membranes composed of palmitic acid with cholesterol (POPC/CHOL) and without cholesterol (POPC) were also used. The results from these experiments are shown in the table below.
| Material | 15 (°C) | 25 (°C) | 35 (°C) | 45 (°C) |
|---|---|---|---|---|
| Lens lipids | 15 cm/s | 30 cm/s | 65 cm/s | 110 cm/s |
| POPC/CHOL | 15 cm/s | 30 cm/s | 60 cm/s | 95 cm/s |
| POPC | 55 cm/s | 100 cm/s | 155 cm/s | 280 cm/s |
| Water | 45 cm/s | 55 cm/s | 65 cm/s | 75 cm/s |
B. Represent these data graphically. The axes should be labeled, and different symbols should be used to plot data for each material.
C. Analyze the data by comparing transport of oxygen through the biological membrane, water, and the synthetic membranes. Consider both membrane composition and temperature in your analysis.
The plasma membrane separates the interior and the exterior of the cell. A potential to do work is established by defining regions inside and outside the cell with different concentrations of key molecules and net charge. In addition to the membrane defining the cell boundary, eukaryotic cells have internal membranes.
D. Explain how internal membranes significantly increase the functional capacity of the cells of eukaryotes relative to those of prokaryotes.
3.3 Lipids
Proteins are polymers whose sub-components are amino acids connected by peptide bonds. The carboxylic acid carbon, O = C – OH, of one amino acid can form a bond with the amine, NH2, of another amino acid. In the formation of this peptide bond, the amine replaces the OH to form O = C – NH2. The other product of this reaction is water, H2O.
Amino acids can be synthesized in the laboratory from simpler molecules of ammonia (NH3), water (H2O), methane (CH4), and hydrogen (H2) if energy is provided by processes that simulate lightning strikes or volcanic eruptions (Miller, Science, 117, 1953; Johnson et al., Science, 322, 2008).
A. The synthesis of amino acids in solutions under laboratory conditions consistent with early Earth was a step toward an explanation of how life began. Pose a question that should have been asked but was not until 2014 (Parker et al., Angewandte Chemie, 53, 2014), when these solutions that had been stored in a refrigerator were analyzed.
The diversity and complexity of life begins in the variety of sequences of the 20 common amino acids.
B. Apply mathematical reasoning to explain the source of biocomplexity by calculating the possible variations in a polymer composed of just three amino acids.
Polarity in a bond between atoms occurs when electrons are distributed unequally. Polarity in a molecule also is caused by charge asymmetry. Life on Earth has evolved within a framework of water, H2O, one of the most polar molecules. The polarities of the amino acids that compose a protein determine the properties of the polymer.
The electric polarity of an amino acid in an aqueous solution depends on the pH of the solution. Here are three forms of the general structure of an amino acid.
C. Qualitatively predict the relationship between solution pH and the form of the amino acid for three solutions of pH: pH 7, pH = 7, and pH > 7.
The properties of proteins are determined by interactions among the amino acids in the peptide-bonded chain. The protein subcomponents, especially amino R (variable) groups, can interact with very strong charge-charge forces, with attractive forces between groups of atoms with opposite polarities and with repulsive forces between groups of atoms with the same or no polarity. Attractive polar forces often arise between molecules through interactions between oxygen and hydrogen atoms or between nitrogen and hydrogen atoms.
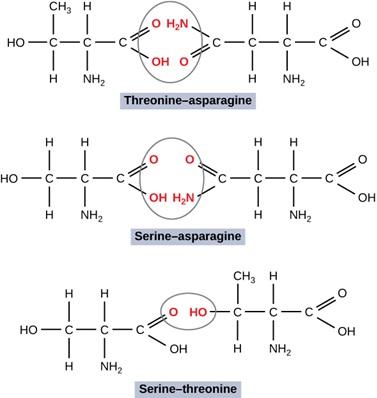
D. Consider particular orientations of pairs of three different amino acids. Predict the relative strength of attractive interaction of all pairs; rank them and provide your reasoning.
In an amino acid, the atoms attached to the α carbon are called the R group.
Interactions between R groups of a polypeptide give three-dimensional structure to the one-dimensional, linear sequence of amino acids in a polypeptide.
E. Construct an explanation for the effect of R-group interactions on the properties of a polymer with drawings showing molecular orientations with stronger and weaker polar forces between R groups on asparagine and threonine and between asparagine and alanine.
3.4 Proteins
The nucleobase part of deoxyribonucleic acid encodes information in each component in the sequence making up the polymer. There are five nucleobases that are commonly represented by only a single letter: A (adenine), C (cytosine), G (guanine), T (thymine), and U (uracil). These molecules form a bond with the 1-carbon of deoxyribose. In this problem, we need to look at the molecules in slightly more detail so that you can development the ability to explain why DNA, and sometimes RNA, is the primary source of heritable information.
Edwin Chargaff and his team isolated nucleobases from salmon sperm and determined the fraction of each (Chargaff et al., Journal of Biological Chemistry, 192, 1951). Experiments in which the fraction of all four nucleobases was determined are shown. Also shown are averages as two standard deviations and the sum of total fractions for each experiment. Precision is calculated with each average.
Shown below are the chemical structures of these four nucleobases. In these structures, the nitrogen that attaches to the 2-deoxyribose, 5-phosphate polymer is indicated as N*. The partial charges of particular atoms are indicated with δ+ and δ-.
A. Analyze Chargaff’s data in terms of the partial charges on these molecules to show how molecular interactions affect the function of these molecules in the storage and retrieval of biological information.
| Experiment | Adenine | Guanine | Cytosine | Thymine | Total |
|---|---|---|---|---|---|
| 5 | 0.28 | 0.20 | 0.21 | 0.27 | 0.96 |
| 6 | 0.30 | 0.22 | 0.20 | 0.29 | 1.01 |
| 7 | 0.27 | 0.18 | 0.19 | 0.25 | 0.89 |
| 8 | 0.28 | 0.21 | 0.20 | 0.27 | 0.96 |
| 11 | 0.29 | 0.18 | 0.20 | 0.27 | 0.94 |
| 12 | 0.28 | 0.21 | 0.19 | 0.26 | 0.94 |
| 13 | 0.30 | 0.21 | 0.20 | 0.30 | 1.01 |
| Average | 0.29±0.02 | 0.20±0.03 | 0.20±0.01 | 0.27±0.02 | 0.96±0.08 |
The interactions between nucleobase molecules are strong enough to produce the association of pairs observed in Chargaff’s data. However, these pairs are bonded by much weaker hydrogen bonds, chemical bonds within the molecules.
Demonstrating understanding of the replication of DNA requires the ability to explain how the two polymer strands of the double helix interact and grow. To retrieve information from DNA, the strands must be separated. The proteins that perform that task interact with the polymer without forming new chemical bonds. In their paper (Watson and Crick, Nature, 3, 1953) announcing the structure of the polymer that we consider in this problem, Watson and Crick stated, “It has not escaped our notice that the specific pairing we have postulated immediately suggests a possible copying mechanism for the genetic material.”
Eschenmoser and Lowenthal (Chemical Society Reviews, 21, 1992) asked why the 5-carbon sugar ribose is used in DNA when the 6-carbon sugar glucose is so common in biological systems. To answer the question, they synthesized polymeric chains of artificial DNA using glucose. They discovered that the strength of the interaction between pairs of nucleobases increased in the DNA with glucose. Paired strands of hexose-based polymers were more stable.
The AP Biology Curriculum Framework (College Board, 2012) states, “The double-stranded structure of DNA provides a simple and elegant solution for the transmission of heritable information to the next generation; by using each strand as a template, existing information can be preserved and duplicated with high fidelity within the replication process. However, the process of replication is imperfect….”
B. Based on the findings of Eschenmoser and Lowenthal, why didn’t DNA evolve to use glucose rather than hexose? What does this have to do with the idea that “replication is imperfect” in DNA? Thoroughly explain your answers.