Learning Objectives
Learning Objectives
In this section, you will explore the following questions:
- What are the basic stages in the biogeochemical cycles of water, nitrogen, phosphorus, and sulfur?
- How have human activities impacted these biogeochemical cycles, and what are the potential consequences for Earth?
Connection for AP® Courses
Connection for AP® Courses
As we learned in Energy Flow through Ecosystems, energy takes a one-way path (flows directionally) through the trophic levels in an ecosystem. However, the matter that comprises living organisms is conserved and recycled through what are referred to as biogeochemical cycles. The six most common elements associated with organic molecules—carbon, nitrogen, hydrogen, oxygen, phosphorus, and sulfur—take a variety of chemical forms and may exist for long periods in Earth’s atmosphere, on land, in water, or beneath our planet’s surface. Geologic processes, including weathering and erosion, play a role in this recycling of materials from the environment to living organisms. For the purpose of AP®, you do not need to know the details of every biogeochemical cycle, though some details of those cycles are covered in this section.
Information presented and the examples highlighted in the section support concepts outlined in Big Idea 2 and Big Idea 4 of the AP® Biology Curriculum Framework. The AP® Learning Objectives listed in the Curriculum Framework provide a transparent foundation for the AP® Biology course, an inquiry-based laboratory experience, instructional activities, and AP® exam questions. A learning objective merges required content with one or more of the seven science practices.
| Big Idea 2 | Biological systems utilize free energy and molecular building blocks to grow, to reproduce, and to maintain dynamic homeostasis. |
| Enduring Understanding 2.A | Growth, reproduction and maintenance of living systems require free energy and matter. |
| Essential Knowledge | 2.A.3 Organisms must exchange matter with the environment to grow, reproduce and maintain organization. |
| Science Practice | 1.1 The student can create representations and models of natural or man-made phenomena and systems in the domain. |
| Science Practice | 1.4 The student can use representations and models to analyze situations or solve problems qualitatively and quantitatively. |
| Learning Objective | 2.9 The student is able to represent graphically or model quantitatively the exchange of molecules between an organism and its environment, and the subsequent use of these molecules to build new molecules that facilitate dynamic homeostasis, growth and reproduction. |
| Big Idea 4 | Biological systems interact, and these systems and their interactions possess complex properties. |
| Enduring Understanding 4.A | Interactions within biological systems lead to complex properties. |
| Essential Knowledge | 4.A.6 Interactions among living systems and with their environment result in the movement of matter and energy. |
| Science Practice | 1.4 The student can use representations and models to analyze situations or solve problems qualitatively and quantitatively. |
| Learning Objective | 4.15 The student is able to use visual representations to analyze situations or solve problems qualitatively to illustrate how interactions among living systems and with their environment results in the movement of matter and energy. |
Water contains hydrogen and oxygen, which is essential to all living processes. The hydrosphere is the area of the Earth where water movement and storage occurs, such as liquid water on the surface and beneath the surface or frozen (rivers, lakes, oceans, groundwater, polar ice caps, and glaciers), and as water vapor in the atmosphere. Carbon is found in all organic macromolecules and is an important constituent of fossil fuels. Nitrogen is a major component of our nucleic acids and proteins and is critical to human agriculture. Phosphorus, a major component of nucleic acid (along with nitrogen), is one of the main ingredients in artificial fertilizers used in agriculture and their associated environmental impacts on our surface water. Sulfur, critical to the 3–D folding of proteins (as in disulfide binding), is released into the atmosphere by the burning of fossil fuels, such as coal.
The cycling of these elements is interconnected. For example, the movement of water is critical for the leaching of nitrogen and phosphate into rivers, lakes, and oceans. Furthermore, the ocean itself is a major reservoir for carbon. Thus, mineral nutrients are cycled, either rapidly or slowly, through the entire biosphere, from one living organism to another, and between the biotic and abiotic world.
Link to Learning
Head to this website to learn more about biogeochemical cycles.
- There is a variable amount of each element on Earth.
- A reservoir is where elements remain through time.
- Only external energy sources drive movement of elements.
- Geochemical cycles are characterized by the movement of elements.
The Water (Hydrologic) Cycle
The Water (Hydrologic) Cycle
Water is the basis of all living processes. The human body is more than 1/2 water and human cells are more than 70 percent water. Thus, most land animals need a supply of fresh water to survive. However, when examining the stores of water on Earth, 97.5 percent of it is non-potable salt water (Figure 37.13). Of the remaining water, 99 percent is locked underground as water or as ice. Thus, less than 1 percent of fresh water is easily accessible from lakes and rivers. Many living things, such as plants, animals, and fungi, are dependent on the small amount of fresh surface water supply, a lack of which can have massive effects on ecosystem dynamics. Humans, of course, have developed technologies to increase water availability, such as digging wells to harvest groundwater, storing rainwater, and using desalination to obtain drinkable water from the ocean. Although this pursuit of drinkable water has been ongoing throughout human history, the supply of fresh water is still a major issue in modern times.
Water cycling is extremely important to ecosystem dynamics. Water has a major influence on climate and, thus, on the environments of ecosystems, some located on distant parts of the Earth. Most of the water on Earth is stored for long periods in the oceans, underground, and as ice. Figure 37.14 illustrates the average time that an individual water molecule may spend in the Earth’s major water reservoirs. Residence time is a measure of the average time an individual water molecule stays in a particular reservoir. A large amount of the Earth’s water is locked in place in these reservoirs as ice, beneath the ground, and in the ocean, and, thus, is unavailable for short-term cycling (only surface water can evaporate).
There are various processes that occur during the cycling of water, shown in Figure 37.15. These processes include the following:
- evaporation/sublimation
- condensation/precipitation
- subsurface water flow
- surface runoff/snowmelt
- streamflow
The water cycle is driven by the sun’s energy as it warms the oceans and other surface waters. This leads to the evaporation (water to water vapor) of liquid surface water and the sublimation (ice to water vapor) of frozen water, which deposits large amounts of water vapor into the atmosphere. Over time, this water vapor condenses into clouds as liquid or frozen droplets and is eventually followed by precipitation (rain or snow), which returns water to the Earth’s surface. Rain eventually permeates into the ground, where it may evaporate again if it is near the surface, flow beneath the surface, or be stored for long periods. More easily observed is surface runoff: the flow of fresh water either from rain or melting ice. Runoff can then make its way through streams and lakes to the oceans or flow directly to the oceans themselves.
Link to Learning
Head to this website to learn more about the world’s fresh water supply.
- Humans utilize water from oceans, which is the most common ecosystem.
- Humans utilize freshwater, which is the rarest ecosystem.
- Humans utilize freshwater, which is the most common ecosystem.
- Humans utilize water from oceans, which is the rarest ecosystem.
Rain and surface runoff are major ways in which minerals, including carbon, nitrogen, phosphorus, and sulfur, are cycled from land to water. The environmental effects of runoff will be discussed later as these cycles are described.
The Nitrogen Cycle
The Nitrogen Cycle
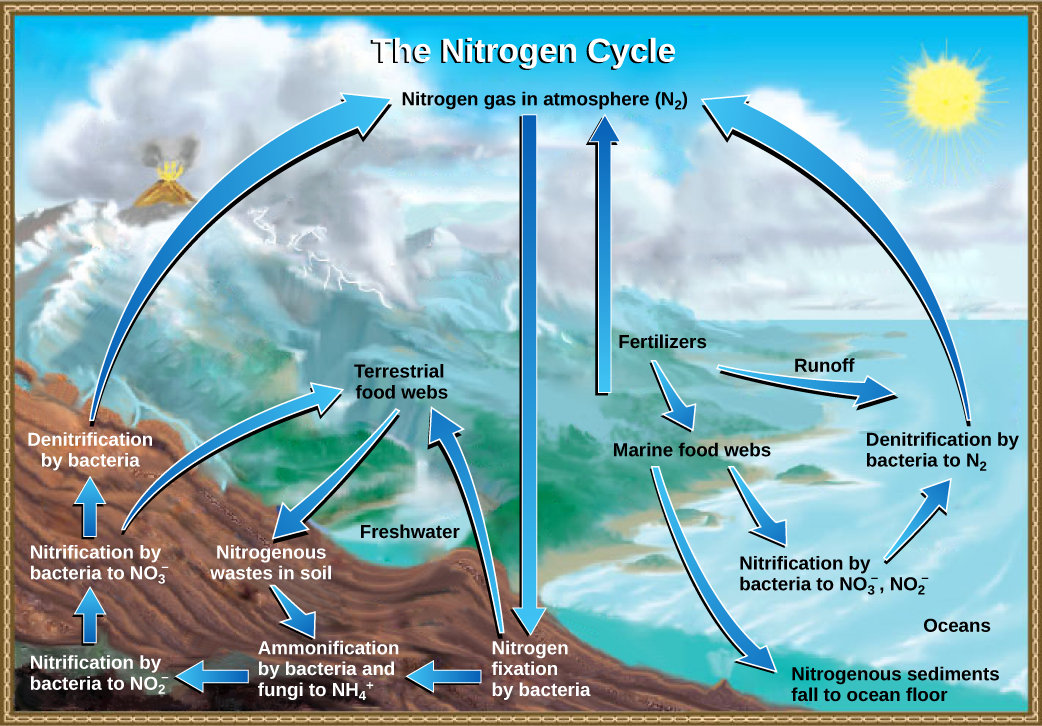
Getting nitrogen into the living world is difficult. Plants and phytoplankton are not equipped to incorporate nitrogen from the atmosphere (which exists as tightly bonded, triple covalent N2) even though this molecule comprises approximately 78 percent of the atmosphere. Nitrogen enters the living world via free-living and symbiotic bacteria, which incorporate nitrogen into their macromolecules through nitrogen fixation (conversion of N2). Cyanobacteria live in most aquatic ecosystems where sunlight is present; they play a key role in nitrogen fixation. Cyanobacteria are able to use inorganic sources of nitrogen to fix nitrogen. Rhizobium bacteria live symbiotically in the root nodules of legumes (such as peas, beans, and peanuts) and provide them with the organic nitrogen they need. Free-living bacteria, such as Azotobacter, are also important nitrogen fixers.
Organic nitrogen is especially important to the study of ecosystem dynamics since many ecosystem processes, such as primary production and decomposition, are limited by the available supply of nitrogen. As shown in Figure 37.18, the nitrogen that enters living systems by nitrogen fixation is successively converted from organic nitrogen back into nitrogen gas by bacteria. This process occurs in three steps in terrestrial systems: ammonification, nitrification, and denitrification. First, the ammonification process converts nitrogenous waste from living animals or from the remains of dead animals into ammonium (NH4+) by certain bacteria and fungi. Second, the ammonium is converted to nitrites (NO2−) by nitrifying bacteria, such as Nitrosomonas, through nitrification. Subsequently, nitrites are converted to nitrates (NO3−) by similar organisms. Third, the process of denitrification occurs, whereby bacteria, such as Pseudomonas and Clostridium, convert the nitrates into nitrogen gas, allowing it to re-enter the atmosphere.
Visual Connection
- Ammonification converts organic nitrogenous matter from living organisms into ammonium (NH4+).
- Denitrification by bacteria converts nitrates (NO3-) to nitrogen gas (N2).
- Nitrification by bacteria converts nitrates (NO3-) to nitrites (NO2-).
- Nitrogen fixing bacteria convert nitrogen gas (N2) into organic compounds.
Human activity can release nitrogen into the environment by two primary means: the combustion of fossil fuels, which releases different nitrogen oxides, and by the use of artificial fertilizers in agriculture, which are then washed into lakes, streams, and rivers by surface runoff. Atmospheric nitrogen is associated with several effects on Earth’s ecosystems including the production of acid rain (as nitric acid, HNO3) and greenhouse gas (as nitrous oxide, N2O) potentially causing climate change. A major effect from fertilizer runoff is saltwater and freshwater eutrophication, a process whereby nutrient runoff causes the excess growth of microorganisms, depleting dissolved oxygen levels and killing ecosystem fauna.
A similar process occurs in the marine nitrogen cycle, where the ammonification, nitrification, and denitrification processes are performed by marine bacteria. Some of this nitrogen falls to the ocean floor as sediment, which can then be moved to land in geologic time by uplift of the Earth’s surface and thereby incorporated into terrestrial rock. Although the movement of nitrogen from rock directly into living systems has been traditionally seen as insignificant compared with nitrogen fixed from the atmosphere, a recent study showed that this process may indeed be significant and should be included in any study of the global nitrogen cycle.
Science Practice Connection for AP® Courses
Think About It
What is the process of nitrogen fixation and how does it relate to crop rotation in agriculture?
The Phosphorus Cycle
The Phosphorus Cycle
Phosphorus is an essential nutrient for living processes; it is a major component of nucleic acid and phospholipids, and, as calcium phosphate, makes up the supportive components of our bones. Phosphorus is often the limiting nutrient (necessary for growth) in aquatic ecosystems (Figure 37.19).
Phosphorus occurs in nature as the phosphate ion (PO43−). In addition to phosphate runoff as a result of human activity, natural surface runoff occurs when it is leached from phosphate-containing rock by weathering, thus sending phosphates into rivers, lakes, and the ocean. This rock has its origins in the ocean. Phosphate-containing ocean sediments form primarily from the bodies of ocean organisms and from their excretions. However, in remote regions, volcanic ash, aerosols, and mineral dust may also be significant phosphate sources. This sediment then is moved to land over geologic time by the uplifting of areas of the Earth’s surface.
Phosphorus is also reciprocally exchanged between phosphate dissolved in the ocean and marine ecosystems. The movement of phosphate from the ocean to the land and through the soil is extremely slow, with the average phosphate ion having an oceanic residence time between 20,000 and 100,000 years.
Excess phosphorus and nitrogen that enters these ecosystems from fertilizer runoff and from sewage causes excessive growth of microorganisms and depletes the dissolved oxygen, which leads to the death of many ecosystem fauna, such as shellfish and finfish. This process is responsible for dead zones in lakes and at the mouths of many major rivers (Figure 37.19).
A dead zone is an area within a freshwater or marine ecosystem where large areas are depleted of their normal flora and fauna; these zones can be caused by eutrophication, oil spills, dumping of toxic chemicals, and other human activities. The number of dead zones has been increasing for several years, and more than 400 of these zones were present as of 2008. One of the worst dead zones is off the coast of the United States in the Gulf of Mexico, where fertilizer runoff from the Mississippi River basin has created a dead zone of over 8463 square miles. Phosphate and nitrate runoff from fertilizers also negatively affect several lake and bay ecosystems including the Chesapeake Bay in the eastern United States.
Everyday Connection
Chesapeake Bay
The Chesapeake Bay has long been valued as one of the most scenic areas on Earth; it is now in distress and is recognized as a declining ecosystem. In the 1970s, the Chesapeake Bay was one of the first ecosystems to have identified dead zones, which continue to kill many fish and bottom-dwelling species, such as clams, oysters, and worms. Several species have declined in the Chesapeake Bay due to surface water runoff containing excess nutrients from artificial fertilizer used on land. The source of the fertilizers (with high nitrogen and phosphate content) is not limited to agricultural practices. There are many nearby urban areas and more than 150 rivers and streams empty into the bay that are carrying fertilizer runoff from lawns and gardens. Thus, the decline of the Chesapeake Bay is a complex issue and requires the cooperation of industry, agriculture, and everyday homeowners.
Of particular interest to conservationists is the oyster population; it is estimated that more than 200,000 acres of oyster reefs existed in the bay in the 1700s, but that number has now declined to only 36,000 acres. Oyster harvesting was once a major industry for Chesapeake Bay, but it declined 88 percent between 1982 and 2007. This decline was due not only to fertilizer runoff and dead zones but also to overharvesting. Oysters require a certain minimum population density because they must be in close proximity to reproduce. Human activity has altered the oyster population and locations, greatly disrupting the ecosystem.
The restoration of the oyster population in the Chesapeake Bay has been ongoing for several years with mixed success. Not only do many people find oysters good to eat, but they also clean up the bay. Oysters are filter feeders, and as they eat, they clean the water around them. In the 1700s, it was estimated that it took only a few days for the oyster population to filter the entire volume of the bay. Today, with changed water conditions, it is estimated that the present population would take nearly a year to do the same job.
Restoration efforts have been ongoing for several years by non-profit organizations, such as the Chesapeake Bay Foundation. The restoration goal is to find a way to increase population density so the oysters can reproduce more efficiently. Many disease-resistant varieties (developed at the Virginia Institute of Marine Science for the College of William and Mary) are now available and have been used in the construction of experimental oyster reefs. Efforts to clean and restore the bay by Virginia and Delaware have been hampered because much of the pollution entering the bay comes from other states, which stresses the need for inter-state cooperation to gain successful restoration.
The new, hearty oyster strains have also spawned a new and economically viable industry—oyster aquaculture—which not only supplies oysters for food and profit, but also has the added benefit of cleaning the bay.
How did fertilizer runoff produce a dead zone in the Chesapeake Bay?
- Excess nitrogen from fertilizer decreases microbial growth, depleting dissolved oxygen in water, thereby killing the fauna of the ecosystem.
- Fertilizer runoff decreases the carbon dioxide concentration in water, thereby killing fauna of the ecosystem.
- Fertilizer runoff produces a dead zone in the Chesapeake Bay by increasing oxygen concentration in the ecosystem.
- Excess nitrogen from fertilizer increases microbial growth, depleting dissolved oxygen in water, thereby killing the fauna of the ecosystem.
The Sulfur Cycle
The Sulfur Cycle
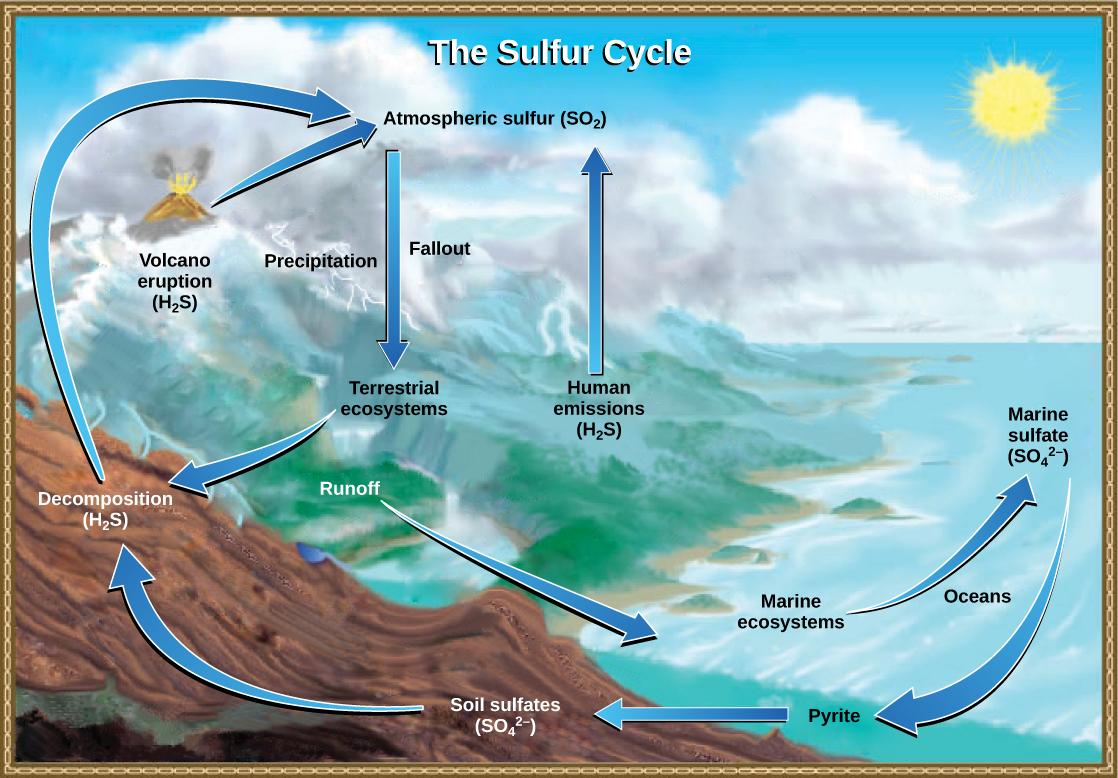
Sulfur is an essential element for the macromolecules of living things. As a part of the amino acid cysteine, it is involved in the formation of disulfide bonds within proteins, which help to determine their 3-D folding patterns, and hence their functions. As shown in Figure 37.21, sulfur cycles between the oceans, land, and atmosphere. Atmospheric sulfur is found in the form of sulfur dioxide (SO2) and enters the atmosphere in three ways: from the decomposition of organic molecules, from volcanic activity and geothermal vents, and from the burning of fossil fuels by humans.
On land, sulfur is deposited in four major ways: precipitation, direct fallout from the atmosphere, rock weathering, and geothermal vents (Figure 37.22). Atmospheric sulfur is found in the form of sulfur dioxide (SO2), and as rain falls through the atmosphere, sulfur is dissolved in the form of weak sulfuric acid (H2SO4). Sulfur can also fall directly from the atmosphere in a process called fallout. Also, the weathering of sulfur-containing rocks releases sulfur into the soil. These rocks originate from ocean sediments that are moved to land by the geologic uplifting of ocean sediments. Terrestrial ecosystems can then make use of these soil sulfates (SO4–), and upon the death and decomposition of these organisms, release the sulfur back into the atmosphere as hydrogen sulfide (H2S) gas.
Sulfur enters the ocean via runoff from land, from atmospheric fallout, and from underwater geothermal vents. Some ecosystems (Figure 37.9) rely on chemoautotrophs using sulfur as a biological energy source. This sulfur then supports marine ecosystems in the form of sulfates.
Human activities have played a major role in altering the balance of the global sulfur cycle. The burning of large quantities of fossil fuels, especially from coal, releases larger amounts of hydrogen sulfide gas into the atmosphere. As rain falls through this gas, it creates the phenomenon known as acid rain. Acid rain is corrosive rain caused by rainwater falling to the ground through sulfur dioxide gas, turning it into weak sulfuric acid, which causes damage to aquatic ecosystems. Acid rain damages the natural environment by lowering the pH of lakes, which kills many of the resident fauna; it also affects the man-made environment through the chemical degradation of buildings. For example, many marble monuments, such as the Lincoln Memorial in Washington, DC, have suffered significant damage from acid rain over the years. These examples show the wide-ranging effects of human activities on our environment and the challenges that remain for our future.
Link to Learning
Click this link to learn more about global climate change.
- The greenhouse effect is reduced due to human activities.
- Human activities strengthen the greenhouse effect by trapping more heat in the atmosphere.
- Human activities decrease the release of carbon dioxide gas, thereby strengthening the greenhouse effect.
- Human activities cause less heat to be trapped in the atmosphere and decrease the temperature.
References
References
Morford, S. L., Houlton, B. Z., & Dahlgren, R. A. (2011, Sept. 1). Increased forest ecosystem carbon and nitrogen storage from nitrogen rich bedrock. Nature 477(7362), 78–81.
The Carbon Cycle
The Carbon Cycle
Carbon is the second most abundant element in living organisms. Carbon is present in all organic molecules, and its role in the structure of macromolecules is of primary importance to living organisms. Carbon compounds contain especially high energy, particularly those derived from fossilized organisms, mainly plants, which humans use as fuel. Since the 1800s, the number of countries using massive amounts of fossil fuels has increased. Since the beginning of the Industrial Revolution, global demand for the Earth’s limited fossil fuel supplies has risen; therefore, the amount of carbon dioxide in our atmosphere has increased. This increase in carbon dioxide has been associated with climate change and other disturbances of the Earth’s ecosystems and is a major environmental concern worldwide. Thus, the carbon footprint is based on how much carbon dioxide is produced and how much fossil fuel countries consume.
The carbon cycle is most easily studied as two interconnected sub-cycles: one dealing with rapid carbon exchange among living organisms and the other dealing with the long-term cycling of carbon through geologic processes. The entire carbon cycle is shown in Figure 37.16.
Link to Learning
Click this link to read information about the United States Carbon Cycle Science Program.
- Carbon sources, such as burning fossil fuels, produce carbon while carbon sinks, such as oceans, absorb carbon.
- Carbon sources, such as volcanic activity, absorb carbon while carbon sinks, such as vegetation, produce carbon.
- Carbon sources, such as vegetation, produce carbon while carbon sinks, such as volcanic activity, absorb carbon.
- Carbon sources, such as volcanic activity, produce carbon while carbon sinks, such as burning fossil fuels, absorb carbon.
The Biological Carbon Cycle
Living organisms are connected in many ways, even between ecosystems. A good example of this connection is the exchange of carbon between autotrophs and heterotrophs within and between ecosystems by way of atmospheric carbon dioxide. Carbon dioxide is the basic building block that most autotrophs use to build multi-carbon, high energy compounds, such as glucose. The energy harnessed from the sun is used by these organisms to form the covalent bonds that link carbon atoms together. These chemical bonds thereby store this energy for later use in the process of respiration. Most terrestrial autotrophs obtain their carbon dioxide directly from the atmosphere, while marine autotrophs acquire it in the dissolved form (carbonic acid, H2CO3−). However carbon dioxide is acquired, a by-product of the process is oxygen. The photosynthetic organisms are responsible for depositing approximately 21 percent oxygen content of the atmosphere that we observe today.
Heterotrophs and autotrophs are partners in biological carbon exchange (especially the primary consumers, largely herbivores). Heterotrophs acquire the high-energy carbon compounds from the autotrophs by consuming them, and breaking them down by respiration to obtain cellular energy, such as ATP. The most efficient type of respiration, aerobic respiration, requires oxygen obtained from the atmosphere or dissolved in water. Thus, there is a constant exchange of oxygen and carbon dioxide between the autotrophs (which need the carbon) and the heterotrophs (which need the oxygen). Gas exchange through the atmosphere and water is one way that the carbon cycle connects all living organisms on Earth.
The Biogeochemical Carbon Cycle
The movement of carbon through the land, water, and air is complex, and in many cases, it occurs much more slowly geologically than as seen between living organisms. Carbon is stored for long periods in what are known as carbon reservoirs, which include the atmosphere, bodies of liquid water (mostly oceans), ocean sediment, soil, land sediments (including fossil fuels), and the Earth’s interior.
As stated, the atmosphere is a major reservoir of carbon in the form of carbon dioxide and is essential to the process of photosynthesis. The level of carbon dioxide in the atmosphere is greatly influenced by the reservoir of carbon in the oceans. The exchange of carbon between the atmosphere and water reservoirs influences how much carbon is found in each location, and each one affects the other reciprocally. Carbon dioxide (CO2) from the atmosphere dissolves in water and combines with water molecules to form carbonic acid, and then it ionizes to carbonate and bicarbonate ions (Figure 37.17)
The equilibrium coefficients are such that more than 90 percent of the carbon in the ocean is found as bicarbonate ions. Some of these ions combine with seawater calcium to form calcium carbonate (CaCO3), a major component of marine organism shells. These organisms eventually form sediments on the ocean floor. Over geologic time, the calcium carbonate forms limestone, which comprises the largest carbon reservoir on Earth.
On land, carbon is stored in soil as a result of the decomposition of living organisms (by decomposers) or from weathering of terrestrial rock and minerals. This carbon can be leached into the water reservoirs by surface runoff. Deeper underground, on land and at sea, are fossil fuels: the anaerobically decomposed remains of plants that take millions of years to form. Fossil fuels are considered a non-renewable resource because their use far exceeds their rate of formation. A non-renewable resource, such as fossil fuel, is either regenerated very slowly or not at all. Another way for carbon to enter the atmosphere is from land (including land beneath the surface of the ocean) by the eruption of volcanoes and other geothermal systems. Carbon sediments from the ocean floor are taken deep within the Earth by the process of subduction: the movement of one tectonic plate beneath another. Carbon is released as carbon dioxide when a volcano erupts or from volcanic hydrothermal vents.
Carbon dioxide is also added to the atmosphere by the animal husbandry practices of humans. The large numbers of land animals raised to feed the Earth’s growing population results in increased carbon dioxide levels in the atmosphere due to farming practices and the respiration and methane production. This is another example of how human activity indirectly affects biogeochemical cycles in a significant way. Although much of the debate about the future effects of increasing atmospheric carbon on climate change focuses on fossils fuels, scientists take natural processes, such as volcanoes and respiration, into account as they model and predict the future impact of this increase.
Disclaimer
This section may include links to websites that contain links to articles on unrelated topics. See the preface for more information.